How is aluminium produced?
|
Alumina is the most widespread metal in nature: making up about 8.8% of the earth’s crust. Due to its chemical reactivity, it almost doesn’t exist in free form. Contrary to popular opinion, aluminium mines do not exist, and only a few minerals and rocks containing aluminium are suitable for industrial production.
Aluminium is mainly produced from bauxite. Over 90% of the world’s bauxite resources are concentrated on the tropical and sub-tropical belt in Australia, Guinea, Jamaica, Surinam, Brazil, and India.
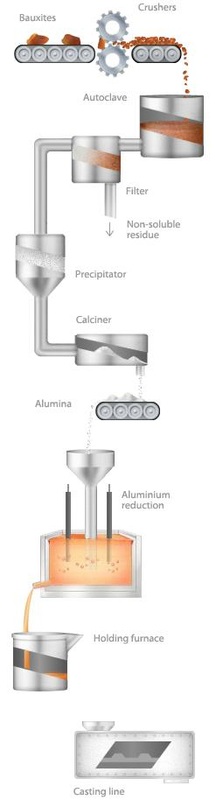
In Russia there are also nepheline ore deposits located on the Kola Peninsula and in the Kemerovo Region. As a result of nepheline processing, significant volumes of by-products are generated including calcined soda, potash, fertilizers, and cement. Alumina — or aluminium oxide (Al2O3), is produced from extracted ore. Despite its name, it has nothing to do with clay or black soil but resembles a flour or very white sand. Alumina is then transformed into aluminium through electrolytic reduction. One tonne of aluminium is produced from every two tonnes of alumina. Bauxite consist of 40-60% alumina, as well as earth silicon, ferrous oxide, and titanium dioxide. To separate pure alumina, the Bayer process is applied. First, the ore is heated in an autoclave with caustic soda. It is then cooled and a solid residue — «red mud» — is separated from the liquid. Aluminium hydroxide is then extracted from this solution and calcined to produce pure alumina. The final stage is the reduction of aluminium through the Hall-Heroult process. It is based on the following principle: when the alumina solution is electrolyzed in molten cryolite (Na3AlF6), pure aluminium is produced. The reduction cell bottom serves as a cathode, and coal bars immersed in cryolite serve as anodes. Molten aluminium is deposited under a cryolite solution with 3-5% alumina. During this process, temperatures reach 950°C, considerably higher than the melting point of the metal itself, which is 660°C. In the Hall-Heroult reduction process, coal anodes are consumed very quickly and should be replaced with new ones. This problem can be solved with the renewable Soderberg electrode. It is formed in a special restoration chamber of coke and tar paste, which is fitted into a steel sheet cover which lies open at both ends. The paste is filled into the upper opening when necessary. It is heated before it reaches the cell with melt. Aluminium production technology applies pre-baked anodes, a method used at many European and American aluminium smelters, and characterised by less power consumption and a negative impact on the environment. The anodes are baked in huge gas furnaces and then, having been fixed into holders, are lowered into a furnace. Consumed electrodes are replaced with new ones and remaining ‘butts’ are sent away for recycling. |
Due to higher ecological requirements established in recent years, mitigating hazardous emissions is the main challenge which facilties using Soderberg technology face. Now the problem is being actively solved by implementing colloid anodes made of special colloidal paste, which is thermally resistant. In terms of environmental indicators, this method is equal to the pre-baked anodes technology. The metal is removed from reduction cells and poured into moulds every 24 hours or more. Aluminium production is very energy-intensive. It is for this reason that the most efficient place to construct aluminium smelters is in remote regions, where there is free access to power sources. Sorce: http://www.aluminiumleader.com/en/facts/extraction/ Also see: http://www.hydro.com/en/About-aluminium/How-its-made/ |